Accutane is a medical drug treatment designed to alleviate the condition of acne. Unfortunately, acne affects four out of five people between the ages of 12 and 25. Studies have shown that those individuals who suffer from severe acne will receive positive results using Accutane acne treatments. The question remains, are the risks worth the benefits?
Accutane, otherwise known as Isotretinoin, is prescribed only for severe nodular acne. Cautious physicians will limit the use of the medication to individuals whose emotional and psychological stressors make severe acne as an emotional problem as it is a physical one. (1)
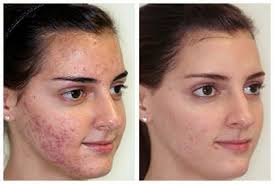
This treatment has several requirements when used, especially in women because the treatments are linked with severe birth defects, miscarriage, premature birth and the death of the baby. The cautionary procedures for men are significantly less than they are for women.
If a woman is pregnant or thinks she may become pregnant, or wants to become pregnant, or is breast-feeding, she should never take Accutane acne treatments. The risks to the baby is so severe that the Federal Government developed an increased risk program especially designed for this drug. (2)
The federal program is called the iPledge program for Accutane acne treatment. The initial design is to ensure that women who are taking Accutane do not get pregnant. The program was instituted in December of 2005 and has many stringent requirements for physicians, patients and pharmacies who are prescribing, taking and filling prescriptions.
Any individual who considers the prescription must be informed of all the risks and agree to carry out all of the instructions in the iPledge program. Women who are in childbearing years and who can become pregnant must first agree to use two separate forms of effective birth control at the same time for one month prior to starting the prescription. They must use two forms during the use of treatment and for one month after stopping the treatment. Women must also agree to have two negative pregnancy tests before receiving their first treatment and a negative pregnancy test before each and every refill of the prescription.
Women must also sign an additional information and consent form which lists the warnings about the risks of potential birth defects if a baby is exposed to Accutane acne treatment. (3)
References:
(1) Acta Dermato-Vernereologica: Study of Psycological Stress, Sebum production and Acne Vulgaris in Adolescents
http://www.ncbi.nlm.nih.gov/pubmed/17340019
(2) Roche Pharmaceuticals: NDA pledge
http://www.fda.gov/downloads/drugs/drugsafety/ucm085812.pdf
(3) March of Dimes: Accutane and Other Retinoids
http://www.marchofdimes.com/pregnancy/alcohol_accutane.html
(4) Drugs.com: Accutane
http://www.drugs.com/accutane.html
| Advertisement | |
|
|



Leave a Reply